What Is Fundamentally Different Between Generating Transgenic Animals Versus Transgenic Plants?
Molecular Farming
The potential of engineered plants as low-input production platforms for big-scale production of pharmaceuticals is an expanse of agile research. Examples of constitute made pharmaceuticals (PMPs) with global markets include human insulin, human serum albumin (HSA) and HIV-neutralizing antibodies. There is a big need for man insulin due to the high incidence of diabetes world-wide, which includes a substantial undersupplied market in Asia. Institute product of insulin could meet this shortfall at a price diabetics in this region could afford (Stoger et al., 2014). Over 500 tons per year of HSA are necessary to treat fetal erythroblastosis, fluid loss due to burn injuries, hypoproteinemia, and ascites acquired by cirrhosis of the liver (Chen et al., 2013). InVitria, a division of Ventria Bioscience has developed Optibumin, a rice-derived HSA that has already been commercialized (He et al., 2011)1. An estimated 5 tons of HIV-neutralizing antibody is needed to supply ten million women with the minimal corporeality necessary to prevent HIV (Shattock and Moore, 2003). Ii publicly funded projects, Pharma-Planta and Hereafter-Pharma take produced HIV-neutralizing antibodies in tobacco and corn seeds for clinical trials. Information technology is hoped their production platform could be used in afflicted areas to produce microbicides "in the region for the region" (Stoger et al., 2014). For all three examples, at that place is a greater demand for these products than there is a supply of them, and this is particularly the example in under-adult countries. The immense scalability of molecular farming could meet the demand at a price that matches the economic situation of the target areas.
Molecular farming also has the potential to enhance the production of pharmaceuticals naturally produced in plants such as the anti-cancer drug Taxol (paclitaxel) and artemisinin, a crucial anti-malarial compound. The plants that synthesize these compounds practise and then in low concentrations and abound slowly resulting in only minute quantities of the desired compound (Buyel, 2018). Taxol was originally extracted from the Pacific yew, Taxus brevifolia, where the bawl from a single 100 year erstwhile tree yields almost 300 mg of Taxol, enough for simply i dose (Horwitz, 1994). Today Taxol is produced past Bristol-Myers Squibb using a semi-synthetic process starting with Taxol intermediates extracted from the Yew tree needles. Using the needles rather than the bark is non-destructive but the Yew tree is all the same slow growing and the intermediates require expensive purification (Howat et al., 2014). Similarly depression amounts of artemisinin – 0.01–1.4% dry weight (DW) – accumulate in sugariness wormwood, Artemisia annua (Ikram and Simonsen, 2017). Engineering the biosynthetic pathways for these compounds into heterologous plants optimized for molecular farming could boost supplies and reduce costs (Wurtzel et al., 2019).
Although the consummate biosynthetic pathway for the production of Taxol hasn't been elucidated the biosynthetic pathway to produce the starting time committed product, taxadiene, has been engineered into Nicotiana benthamiana. The full biosynthetic pathway for artemisinin has as well been engineered into N. tabacum. Both tobacco species are production platforms for molecular farming due to their fast growth and loftier biomass production. The most successful try to biosynthetically produce artemisinin took 2 large sections of the metabolic pathway for artemisinin and genetically engineered them separately into three different cellular compartments (chloroplast, nucleus, and mitochondria). The resulting heterologous expression of artemisinin at ∼0.8 mg/g DW was less than in the native establish, which tin reach 31.4 mg/g DW (Zhang et al., 2009; Malhotra et al., 2016). This can in office be explained by the complexity of the cistron expression and regulation of the biosynthetic pathway (Ikram and Simonsen, 2017). A mg/one thousand comparison also doesn't reflect Due north. tabacum'south faster growth and higher biomass production when compared to A. annua. The genetic engineering of Northward. benthamiana to express a taxadiene synthase gene, which produces taxadiene from geranylgeranyl diphosphate (GGPP), produced 11–27 μg/1000 DW taxadiene. Further suppression of the phytoene synthase gene and add-on of methyl jasmonate increased taxadiene accumulation to 35 μg/grand DW (Hasan et al., 2014). The successful de novo production of taxadiene could lead to the development of a heterologous establish system that biosynthesises Taxol. Future improvements in metabolic engineering could see a breakthrough in how these loftier value compounds are produced.
Using plants for the production of enzymes or other proteins impacts both the prophylactic and the potential activeness of the isolated products. Establish product is besides gratuitous from human pathogens – a major concern in mammalian prison cell culture production systems – and free from endotoxins, which are a take a chance in bacterial systems (Commandeur et al., 2002). Poly peptide glycosylation patterns can be manipulated in plants, including to produce 'humanized' glycosylation patterns (Hanania et al., 2017; Mercx et al., 2017). This is important for circuitous glycoproteins such as monoclonal antibodies or membrane proteins as glycosylation can affect protein stability, subcellular targeting, biological action, and immunogenicity (He et al., 2014). The glycosylation of asparagine or arginine side-chains is similar for plants and mammals until the glycan reaches the Golgi apparatus. In plants the side-chain can be modified by the attachment of an α(1,3)-linked fucose or β(ane,2)-linked xylose, whereas in mammals there tin be the attachment of an α(1,6)-linked fucose, β(1,four)-linked galactose or sialic acid (Gomord et al., 2010). In some cases, institute glycosylation produces proteins with higher pharmacological activity than proteins produced by bacterial or mammalian cells. For example, establish production systems produce taliglucerase alfa, a mannose-terminated glycoprotein for the handling of Gaucher's affliction, where terminal mannose residues are needed to bind to macrophage mannose receptors. In contrast, mammalian cell system product requires post-production glycosylation modifications to expose terminal α-mannose residues (Grabowski et al., 2014). There is, however, the possibility alternative glycosylation will increase the chance of immunogenicity. Several plant production systems have been engineered to give the recombinant poly peptide homo glycosylation patterns (Kallolimath et al., 2016).
Plants can too produce large volumes of industrial compounds. Examples of constitute made industrial compounds (PMIs) include cellulases and amylases for bioethanol product, xylanases to raise brute feed and oxidation/reduction enzymes such as laccases and peroxidases for newspaper manufacturing (Van Der Maarel et al., 2002; Bailey et al., 2004; Clough et al., 2006; Shen et al., 2012; Hood and Requesens, 2014). Currently, bioethanol is produced by using starch derived from corn. To enhance this process Syngenta's genetically modified (GM) corn, Enogen, expresses an α-amylase enzyme, which catalyzes the breakdown of starch into glucose (Que et al., 2014). Corn is also used every bit animate being feed or human being nutrient, meaning that in that location is contest for agronomical space. Found biotechnology could enable utilizing more of the cellulose and hemicellulose to be used to produce biofuels. The The states company Agrivida increased ethanol production past 55% by engineering corn to express cell wall degrading enzymes in planta (Zhang et al., 2011).
Transgenic plants have been developed to be a source of fibrous animal proteins such every bit collagen, keratin, silk, and elastin (Börnke and Broer, 2010). The Israeli biotechnology company CollPlant developed a tobacco line to produce recombinant human being collagen (Stein et al., 2009). Typically, medical collagen comes from animal or human being cadavers which pose an infection risk from prions (Pammer et al., 1998). Additionally, the extraction procedure forms unwanted inter- and intra-molecular bonds, which reduce the solubility and the ability of the collagen to form into more desirable highly structured scaffolds (Zeugolis et al., 2008). Whereas, the plant-derived collagen is cross-link and pathogen costless, so information technology tin can be modified for the desired application.
For maximal scalability and cheap product molecular farming is conducted with field grown crops. A good case report is the institute biotechnology company Space Enzyme, which uses field grown corn to heterologously produce 1.5 million kg of cellulase annually (the amount needed for a 190 million liter per twelvemonth cellulosic biofuel facility). To produce and process field grown corn but $2 one thousand thousand in capital investment – for dry milling and defatting equipment – was required; with $11.7 million per year in operating costs ($7.viii/kg enzyme). In contrast, a microbial fermentation system, which requires tanks and the associated infrastructure, would require $100 million in upfront upper-case letter investment. A further $15 million per year would also be needed in operating costs ($x/kg enzyme)2. However, the economic advantages of using a field grown ingather, must be balanced out past the possibility of the transgene contaminating other crop production.
The Troubled History of Transgene Escape
While molecular farming has the potential to lower the toll of medications and industrially useful compounds, the growth of these technologies is contingent on the containment of the transgenes. Challenges of transgene biocontainment are not just hypothetical; in that location are 2 salient examples of the need for effective containment – the ProdiGene and StarLink affairs (Murphy, 2007). ProdiGene produced a transgenic corn that expressed a vaccine for preventing bacteria-induced diarrhea in pigs, and while the vaccine protein was non-toxic to humans, strict exclusion from the human being food concatenation was required (Hileman, 2003). StarLink's corn crop was genetically engineered with a gene for resistance to the herbicide glufosinate, and it contained a variant of the pest control Bacillus thuringiensis (Bt) poly peptide (Cry9C) – information technology also lacked approving for food use. In 2000, StarLink's transgenic corn contaminated millions of tons of non-transgenic corn throughout the Us. Government officials take said StarLink'due south programmer, Aventis CropScience, failed to ensure farmers kept StarLink corn divide from other varietiesthree. The contaminated corn was recalled for disposal, costing Aventis an estimated $500 million (Murphy, 2007). In 2002, ProdiGene failed to eradicate plants that had seeded from their previous season'southward transgenic corn crop. This led to the contamination of non-transgenic soybeans. ProdiGene's failure to manage their transgenic corn ingather resulted in 12,000 tons of soybean being destroyed. The combined toll to ProdiGene was near $3.v million with an additional US government fine of $250,000 (Thayer, 2002).
The fallout from the ProdiGene and StarLink affairs was lasting. In response the molecular farming manufacture pushed for tighter regulations regarding the blessing process for molecular farming crops (Murphy, 2007). In 2003, the Fauna and Constitute Wellness Inspection Service (APHIS) of the US Section of Agriculture (USDA) introduced the requirement that crops engineered to produce PMIs be grown under permit. Previously, a GM PMI producing crop could be cultivated under notification, which expedited the permitting procedure (Federal Annals [FR], 2005). A full discussion of the interplay between regulation and molecular farming is beyond the scope of this review. Although, it is worth making the betoken that regulatory hurdles remain a bulwark to molecular farming. For example, Syngenta's development of Enogen toll several 100 million dollars, a lot of which was due to it taking almost 6 years to pass USDA's regulatory review process (Wang and Ma, 2012). It is promising though that in 2011 Enogen met USDA's requirements to be fully deregulated. In doing so Enogen became the first constitute genetically engineered for industry to be granted this status (Wang and Ma, 2012). The success of Enogen shows a pathway to the commercialization of a PMI product platform.
Inefficient transgene biocontainment has impacted international trade. Japan and South korea halted imports of corn from the United states during the StarLink corn incident. Exports of wheat to Nihon and South Korea were also briefly stopped in 2013, afterward a GM wheat event MON71800 – developed past Monsanto to be glyphosate-tolerant, was institute growing in a field. Monsanto paid $two.1 million to farmers to compensate the loss of export income and reputational harm, and paid $250,000 to several wheat growers' associationsfour. In 2016, a sis issue (the same DNA was inserted into a different genomic location) – MON71700 – was found to accept contaminated a field in the land of Washington. The 22 plants descend from a field trial conducted by Monsanto from 1998 to 2001v. In both cases the reoccurrence of the GM wheat was unexplained. The precedent of a GM crop re-emerging more than a decade after a trial stokes public concern over food condom and biosecurity. Such concerns will go on to impact the adoption and development of establish biotechnologies (Irish potato, 2007). In gild to foster acceptance of transgenic found product systems there must be proper containment and security at all levels of production.
Importance of Biocontainment
There are concerns from the public and from inside the scientific community that molecular farming could threaten not-GM agronomics, the surroundings, and human health. Without acceptable biocontainment, neighboring not-GM crops or weeds could receive transgenes and transgenic seeds could contaminate seed storage (Mallory-Smith and Sanchez Olguin, 2011; Gressel, 2015). Contamination worries many in the food industry, who are not involved with molecular farming, but could suffer financially and in terms of public confidence if theirs or any other major edible crop became contaminated (Murphy, 2007). Contamination can impact international trade betwixt countries that have legal restrictions on importing transgenic products (Lu, 2003). There are besides environmental concerns stemming from the possibility of crop-to-wild transgene flow. In most cases, the few resulting offspring from ingather × wild crosses will be outcompeted due to being less locally adapted than the wild type (Gressel, 2015) although the transfer of herbicide resistance genes to weeds, including invasive species, could increase the difficulty of eradicating them. It is improbable, only a transgene could also spread from an engineered crop to a weed and then from that weed to another ingather. In this way, weeds that incorporate the transgene could act every bit a reservoir for that transgene allowing spread to not-GM crops.
In some cases, molecular farming could potentially pose a risk of humans or animals being harmed through inadvertent exposure to an unsafe level of recombinant poly peptide (Breyer et al., 2012). The majority of PMPs currently in production, such as antibodies, growth hormone, insulin and near other proteins, are expected to have no pharmacological effect when ingested (Goldstein and Thomas, 2004). Instead the gastro-intestinal tract volition degrade most PMPs to harmless peptides or amino acids. However, many exceptions may be in the future, and some plant pharmaceuticals, such equally oral vaccines, are designed to be active when ingested. In that location is also potential for skin or eye contact and inhalation of the recombinant protein as well as the potential allergenicity of the plant itself (Breyer et al., 2012). The homo wellness threats are heightened by the fact that a plant product could enter the human food or animal feed chain. An consequence that is more likely if the transgenic crop is likewise a food crop, as was seen for ProdiGene.
Also as potentially exposing humans or animals to a harmful compound, contagion can affect the quality of related crops. The N American Miller's Association were concerned that the transgene for amylase expression in Enogen could spread into other corn varieties and result in lower quality tortillas, corn puffs, and staff of life (Waltz, 2011). The advance of agronomics will likely see new crop varieties generating novel products such equally cotton engineered to be red in color. In guild to maintain the phenotypic integrity of transgenic and non-transgenic cultivars constructive biocontainment will be required.
The potential economic, environmental, and health threats from molecular farming tin can be greatly reduced through decision-making the flow of the transgene. Information technology's as well important to betoken out that the level of threat from transgene escape depends on the nature of the contamination. Trace mixing of seed that contains a toxic poly peptide is unlikely to be harmful due to dilution. Even so, the introgression of a transgene, which expresses a toxic protein, into a neighboring crop or weed could seriously contaminate human food or animal feed chains. Although whatsoever contamination, regardless of risk, will likely impact public support for GM agronomics.
Transgene Containment Technologies
Gene menses is a process where the frequency of a gene changes in a population and tin occur through gametes, an organism or groups of organisms moving from one population to another. The potential for in that location to be gene flow into or from a ingather depends on the ingather's pollination strategy, on the size of the crop, seed size and viability, and whether there are compatible species within pollination distance (Mallory-Smith and Sanchez Olguin, 2011). Figure i details the iii master ways that transgenes can spread into the environment. Volunteer plants – plants that have cocky-seeded from a previous flavour'southward ingather – tin can contaminate the next season'southward crop if they are accidentally harvested alongside the intended crop (Michael et al., 2010). Transgenes may also spread in seeds that can be spilled during the harvest and transfer of seed. Lastly, cross-pollination can lead to either transgenes escaping into neighboring plants or introgression from neighboring plants into the transgenic crop (Gressel, 2015). As we are primarily concerned with the movement of genes into another population, pollen transfer is the form of gene period that is of nigh concern.

Figure 1. The iii main pathways for unwanted contamination or gene flow in an agronomical setting; with a list of the genetic biocontainment technologies that could exist used to reduce the possibility of the gene menstruum occurring. (A) Seed dispersal during harvest and transport. (B) Contamination from volunteer plants. (C) Genetic biocontainment can limit pollen-mediated factor flow unidirectionally, where transgenes are prevented from spreading from the transgenic ingather into neighboring plants, and it can operate bidirectionally, where gene menses into the transgenic crop is also limited.
At that place are essentially ii approaches for minimizing gene flow: containment and mitigation. Containment aims to stop the flow of the cistron from the crop and mitigation focuses on preventing the gene from establishing in a pregnant proportion of the population (Gressel, 2015). Containment can be physical or biological. Concrete containment provides a barrier, such as a greenhouse, filters in the lab or isolation distances in the field. There are also efforts to conduct molecular farming underground, e.chiliad., in unused mines, which provide an even higher degree of physical containment6. So far there are no documented cases of physical containment failing in the laboratory or greenhouse (Gressel, 2015). Whereas, the shortcomings of geographic isolation were shown when transgenes from GM glyphosate-resistant creeping bentgrass, Agrostis stolonifera, were constitute in non-agronomic bentgrass upwards to iii.viii km beyond the control area perimeter (Reichman et al., 2006). With the unreliability of geographic isolation in many situations it is preferable to avert the use of crop plants grown for homo or beast consumption.
Alternative plant product platforms accept been developed to reduce the risk of contamination. Some examples of non-nutrient and non-feed crops include tobacco (N. benthamiana), duckweed (Lemna minor), microalgae (Chlamydomonas reinhardtii), and moss (Physcomitrella patens) (Yao et al., 2015). As can be seen from Tabular array 1, tobacco and moss are popular production platforms. The use of these plants prevent introgression of a transgene into a found used for food or feed. If a ingather found is to be used, crops that can be crossed with weedy relatives, such as the sunflower, Helianthus annuus, should be avoided.
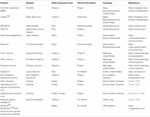
Tabular array ane. Examples of plant made pharmaceuticals.
Sound biocontainment and rapid product of recombinant protein tin be achieved using a transient expression system which does not event in a transgene integrated in the germline. I method to establish a transient expression organization is agroinfiltration where the bacteria Agrobacterium tumefaciens – acting as a vector for the gene of interest – is injected or vacuum infiltrated into leaf cells (Whaley et al., 2011). Another approach is to utilize constitute RNA viruses (Yusibov et al., 2006). Both of these approaches tin be combined, where agroinfiltration is used to evangelize RNA viral vectors into the leaves of a found. This process, called 'magnifection' combines the transfection efficiency of A. tumefaciens, the post-translational modifications of a found and the loftier expression yield obtained with viral vectors (Marillonnet et al., 2005). In all of these approaches the transferred Deoxyribonucleic acid is expressed but not integrated into the germline. The tobacco N. benthamiana is well-nigh often used equally the production platform due to the ease with which information technology can be transformed. Compared to the time it takes to establish a stable transgenic constitute line – 6 months to a year – transient expression systems can produce recombinant protein within iii–5 days (Yao et al., 2015). This is ideal for combating sudden viral epidemics, such as severe astute respiratory syndrome (SARS) or Ebola. Transient expression systems, as a event of not introducing transgenes into germline tissue, don't risk contaminating food through transgene outflow into non-GM crops or their wild relatives (Huafang Lai and Jake Stahnke, 2013). Nevertheless, Agrobacterium infiltration is labor intensive, which was a barrier to transient expression supplying sufficient supplies of an Ebola vaccine (Yao et al., 2015).
Whole-establish production platforms remain bonny due to their scalability merely for some applications in vitro systems are preferable. Electric current in vitro technologies include plant–cell intermission and hairy root cultures. Institute–cell suspensions are typically derived from new tissue formed over a establish callus, which has been cultivated on solidified media. The clumps that easily break apart tin be transferred to liquid media. If a homogenous culture forms, the fermentation of the plant cells can be conducted using similar techniques to fermenting lower eukaryotes (Fischer et al., 1999). Cell break cultures have audio containment and accept a quick development cycle but are a much less scalable product platform, when compared to transgenic plants (Santos et al., 2016). Hairy root cultures are differentiated cultures of transformed roots generated by infection with Agrobacterium rhizogenes (Häkkinen et al., 2014). Hairy root culture tin can be grown with unproblematic defined media like undifferentiated cells, merely information technology has greater genetic stability and it is highly scalable. These features get in suitable for producing pharmaceutical proteins at an industrial-scale (Guillon et al., 2006). However, in vitro techniques require sophisticated and sterile laboratory settings. If the scalability and low-cost potential of plant production of PMPs or PMIs is to be realized, plants need to be grown in fields.
The higher contamination run a risk from growing plants in fields can be reduced by genetic containment which may exploit existing reproductive limitations or introduce them via genetic technology. Many genetic approaches for containing found transgenes have been investigated including cleistogamy, maternal inheritance, gametic transgene excision, synthetic auxotrophy, full sterility, and genetic use restriction technologies (GURT or Terminator). Other genetic containment technologies in evolution could exist extended to plants, such as engineered genetic incompatibility (EGI), genetic recoding and targeted transgene removal (run into Table 2). Many of these technologies piece of work well for specific types of plants and can be enhanced past pairing them with other technologies.

Table two. The important features of genetic biocontainment technologies.
Cleistogamy, where there is cocky-pollination inside a closed flower, is a promising tool to limit gene period. Currently it suffers from some flowers opening, which allows for cross-pollination. Cleistogamy requires that the plant's flower incorporate male and female parts and that there tin can exist self-fertilization. Crops similar rice accept such flowers, only plants that have split up male and female person flowers, like asparagus and spinach, or with unusual flower anatomies, such as corn, aren't suited for cleistogamy. It was found that for imidazolinone herbicide resistant rice a few flowers opened enabling hybridization with weedy rice (Gealy, 2005). To combat this, rice was genetically engineered to enhance the percent of cleistogamous flowers through incorporating the cleistogamous gene, 'superwoman1.' The engineered cultivar, in a variety of plots, had an outcrossing rate of 0.000% compared to the non-engineered cultivar, which ranged from 0.005 to 0.200% (Ohmori et al., 2012). The potential of cleistogamy is limited for GM food product as current practices tend to use college-yielding hybrid rice varieties, which require parental lines that aren't cleistogamous (Gressel, 2015). Withal, this would not exist an issue for the molecular farming of high value compounds where cleistogamy could be used to restrict pollen mediated gene menstruation.
Constructed auxotrophy works by genetically applied science a strain to depend on an externally supplied compound. The dependence can come from deleting essential genes that are needed, for instance, to synthesize amino acids or co-factors that are necessary for crucial biological functions (Moe-Behrens et al., 2013). So far, this approach has plant lilliputian traction for use in plants. There are isolated cases such equally the duckweed Lemna, which has been engineered to be dependent on the add-on of isoleucine through inactivating threonine deaminase expression (Nguyen et al., 2012). However, the genetic redundancy that is a common feature of constitute genomes increases the difficulty in engineering recessive auxotrophic mutations. For almost plants there are likely multiple proteins that catalyze the same reaction, which requires a large number of genetic changes to confer metabolic dependence (Last et al., 1991). The addition of potentially expensive chemicals, in itself a drawback, also requires changes to normal cultivation techniques. Synthetic auxotrophy can also neglect due to introgression of genes from non-transgenic plants, which could restore the knocked out metabolic pathway.
Another approach exploits the maternal inheritance of plastids (e.g., chloroplasts). For the vast majority of higher plants, which display maternal inheritance, transgenes located in the plastid genome are unlikely to be transmitted to other plants by pollination (Maliga, 2004). Plastid engineering has therefore been employed to locate the transgene in the plastid genome, even so, the advance of plastid technology has been stymied by poor transformation protocols for plants other than tobacco. Transformation relies on many essential factors unique to the species and sometimes unique to the cultivar (Lu et al., 2013). There must be detailed knowledge of the plastid genome sequence including the regions in between genes suitable for transgene integration, in that location also needs to exist an optimized DNA delivery system, as well every bit effective antibiotic pick and selectable mark genes. For several years the chloroplast genome sequences have been available for monocots, such as wheat and corn, only the chloroplast hasn't been transformed due to the engineering complexity (Wani et al., 2015). This approach may also exist less efficient than envisioned considering that species that were thought to strictly engage in maternal plastid inheritance notwithstanding had about 0.4% plastid manual via pollen (Avni and Edelman, 1991; Svab and Maliga, 2007). Boosted problems are: proteins expressed in the chloroplast undergo different post-translational modifications, meaning that enzyme function might exist contradistinct (Grabsztunowicz et al., 2017); plastid transformation tin can too be laborious and time-consuming (Ruf et al., 2001).
Total sterility offers a sound footing for genetic biocontainment. Several crops are already sterile or have sterile varieties, such equally cassava (Manihot esculenta), potatoes (Solanum tuberosum), and banana (Musa acuminata) (Celis et al., 2004; Heslop-Harrison and Schwarzacher, 2007; Sayre et al., 2011). Every bit long as the sterility is not leaky, these crops would be safe candidates for molecular farming. A totally sterile plant tin can likewise be engineered by deleting genes that encode for gamete product (Kwit et al., 2011). The downsides are that total sterility requires plants to be vegetatively propagated past either tubers, tissue civilization, cuttings, or artificial seed (Gressel, 2015). Total sterility could exist used with tuber or bulb propagated crops, leafy vegetable crops and forestry. Whereas crops that are harvested for compounds accumulated in seeds would not be candidates for total sterility.
Gametic transgene excision uses a site-specific recombination system to excise a transgene. Currently, the efficiency of the recombinase is quite low, where 99% excision is considered to be high performing (Moon et al., 2011). This level of efficiency is too depression to restrict transgene escape, notwithstanding, it could exist used to excise selectable marking genes used in the engineering of transgenic plants (Hu et al., 2013). Farmers are besides not able to collect seed containing the transgene for future seasons unless the recombinase can exist externally controlled, which alters normal cultivation practices (Ryffel, 2014; Gressel, 2015).
Genetic use restriction technologies were originally developed to prevent farmers from infringing on patents by saving seed. They accept been some of the near controversial GM biotechnologies due to the widespread perception that they were designed to entrench a multinational corporation seed monopoly (Lombardo, 2014). GURTs use a tightly controlled genetic system to regulate the expression of a target gene. There are typically four components to this genetic organisation: the target gene, the target gene's promoter, the trait switch and the genetic switch. The target gene needs to be activated by the promoter. In club to forestall leaky expression from unwanted promoter activity a blocker sequence separates the promoter from the target gene. The blocking sequence can in turn be removed through a cascade beginning with an external input, which will be amplified by the genetic switch. The amplified input becomes a biological betoken that activates the trait switch. The trait switch usually encodes an enzyme, such as a site-specific recombinase that removes the blocker sequence (Lombardo, 2014). Without the blocker sequence at that place can then be transcription and expression of the target gene.
In part due to public opposition GURTs have never been commercialized. Nonetheless, at that place is scope for GURTs to be used for biocontainment. For this, the GURT system would be linked to the transgene, and so that when the GURT is activated there is expression of a disrupter gene that drives cell death. Disrupter genes typically encode for cytotoxins such every bit barnase and ribonuclease A (Mariani et al., 1990; Burgess et al., 2002; Gils et al., 2008; Zhang et al., 2012). There is no show that disrupter genes generate products that are toxic to humans or animals. However, it is possible that the potential health hazard will add together to the already controversial nature of using GURTs (Conner et al., 2003; Gressel, 2010). The other disadvantages to GURT are that it is a more expensive system, requiring exogenous inputs and there is greater difficulty in propagating a GURT crop.
Future Biological Containment Technologies
The biocontainment technologies that have been developed in microbes could in some cases exist extended to plants. Some of these technologies include, genetic recoding, targeted transgene removal and EGI.
Genetic recoding removes every instance of at least one codon for an amino acid in an organism's genome and replaces it with some other. The codon that has been removed tin be replaced with a synonymous codon or it can and then encode for a non-standard amino acid (NSAA) (Mukai et al., 2017). If an essential gene was recoded to require an amino acid not found in nature this would increment the stringency of an auxotrophy. Further, the genetic recoding could create reproductive isolation and cake cistron period with non-recoded organisms due to incompatible genetic codes. Escherichia coli has been recoded and then that the UAG stop codon instead incorporated a NSAA in the cores of essential enzymes. This conferred a dependence on constructed metabolites for proper protein function, such that the bacteria were less capable of mutational escape and metabolic supplementation (Mandell et al., 2015). Following on from this the genetic recoding of plant genomes could confer better biocontainment. Despite the advance of the engineering, nosotros are unlikely to see recoding of higher organisms with ease in the near time to come due to the calibration of changes needed in large genomes.
Some other strategy could exist to precisely remove the engineered genes instead of killing the whole organism. The spread of transgenes from volunteer plants or inadvertent seed dispersal could exist mitigated past using a CRISPR-based organisation to selectively remove the transgene after the desired protein has been produced. In i such method, a genetically encoded device, termed DNAi, responds to a transcriptional input by degrading Dna next to a synthetic CRISPR array. The DNAi system was shown to be non-toxic when carried in Eastward. coli, and when activated it was able to reduce the number of feasible cells by i.ix × 10–8 making it one of the most effective switches for programmed cell death (Caliando and Voigt, 2015). This same mechanism could be engineered so that with the addition of a transcriptional input the transgene is degraded. An advantage of this system is that the removal of the transgene applies little selective pressure toward deactivating the genetic machinery; whereas directing whole organism death selects for mutations that lead to an organism'south survival.
The aforementioned biological containment technologies, with the exceptions of cleistogamy, genetic recoding, and total sterility, don't forbid the flow of genes into the transgenic plant. This is an of import consideration as unwanted gene period can modify important traits in a genetically engineered organism. In society to restrict gene flow in both directions, plants could exist engineered to exist genetically incompatible with related plants such that the hybrid is less fit – this is known as underdominance. The model organism Drosophila melanogaster has been engineered such that engineered-WT hybrids display underdominance. This was accomplished using a genetic construct to encode for two genes: the first encodes for a RNAi knockdown of the WT version of the gene Rpl14; the second gene is a refactored version of Rpl4 such that information technology isn't susceptible to RNAi knockdown. When the engineered organism was mated with WT flies there was a marked fitness reduction in the heterozygotes (Reeves et al., 2014). However, in order to be constructive for biocontainment the underdominance must result in total sterility or death of the hybrids.
An artificial reproductive barrier, where the hybrids are non-viable, has been engineered in Saccharomyces cerevisiae using EGI. This system utilizes programmable transcriptional activators (PTAs) to overexpress a gene leading to lethality. Lethality in the engineered organism is avoided by editing the target sequence of the PTA, such that the PTA is unable to bind and overexpress the factor (Maselko et al., 2017). When there is a cross between the WT and the engineered organism, the PTA targets the WT PTA binding sequence and drives lethal levels of gene expression. Attempts have also been fabricated at amalgam a synthetic species of D. melanogaster, where an bogus reproductive bulwark is engineered, however the goal of consummate genetic isolation wasn't achieved. The principal difficulty proved to be getting strong activation of a lethal gene without the fitness costs associated with broad expression of the transactivating CRISPR mechanism (Waters et al., 2018).
There is an inherent versatility to the utilize of PTAs, and then that lethal overexpression of a target gene could theoretically exist engineered in any sexually reproducing organism (Maselko et al., 2017). Proof of concept has so far only been established in S. cerevisiae. Although, it is conceivable that EGI could exist extended to plants, where it could be used to generate many orthogonal strains of the aforementioned parent species which could each be used equally production platforms for unlike compounds. If interbreeding can be prevented then the phenotypic integrity of transgenic cultivars could be protected. EGI could also exist used to brand constructed auxotrophy more robust by preventing introgression from neighboring plants, which would otherwise compromise the auxotrophy.
Transgene Mitigation Technologies
Even the near stringent containment organisation can fail. Technologies are therefore needed to reduce the chances of a transgene becoming established after escape. Transgenic mitigation involves linking the transgene to genes that confer a selective disadvantage. Weedy traits such as a propensity toward shattering, bolting, and greater height can be targeted (Gressel, 2015). Transgenic mitigation reduced the reproductive fettle of transgenic-weed oilseed rape hybrids. A dwarfing mitigator gene was linked to a herbicide resistance transgene, which reduced the reproductive fitness of the transgenic-weed hybrid to 0.9% of the competing weed's reproductive fitness (Al-Ahmad and Gressel, 2006). However, in that location is the potential for the linkage of the mitigator gene to the transgene to be broken through meiotic crossing over. Additionally, there can be mutation of the mitigator gene so that it ceases to confer the deleterious phenotype. Both of these bug can in some part exist addressed through linking another mitigator gene to the transgene, such that there are mitigating genes either side of the transgene (Gressel, 2015).
Determination
Molecular farming has the potential to lower the cost of medication and industrial enzymes. All the same, in cases where the recombinant protein is potentially toxic, at that place are environmental and human health risks. The introgression of the transgene into a neighboring crop or weed may contaminate food or feed supplies. Any contamination effect, such as in the loftier-contour cases of StarLink and ProdiGene, could jeopardize conviction in molecular farming. For these reasons there must be effective containment of transgenes.
There has been considerable progress in the development of biological containment technologies. For some species such equally rice, cleistogamy could contain gene menses. For tubers and bulb propagated crops total sterility is practical. Simply for many species these technologies aren't applicable. At that place is some hope that technologies like EGI combined with synthetic auxotrophy could incorporate gene menstruum. Further work in this expanse is needed to ensure the condom and widespread adoption of field grown molecular farming crops.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and canonical it for publication.
Funding
This work was funded through the CSIRO Synthetic Biology Future Scientific discipline Platform and a Macquarie University Research Excellence Scholarship.
Conflict of Interest
MM is a co-founder and chief technical officer of NovoClade LLC.
The remaining author declares that the research was conducted in the absence of any commercial or fiscal relationships that could be construed as a potential disharmonize of involvement.
Footnotes
- ^ https://invitria.com/cell-civilization-products/optibumin-lipid-reduced-recombinant-human-albumin-rhsa/
- ^ https://infiniteenzymes.com/engineering-ii/
- ^ https://web.archive.org/web/20070711190925/http://archives.cnn.com/2000/FOOD/news/10/18/conagra.grain.ap/
- ^ https://time.com/3582953/monsanto-wheat-farming-genetically-modified-settlement/
- ^ https://monsanto.com/company/media/statements/statement-gmo-wheat-plants/
- ^ https://www.wired.com/2004/05/drug-farms-forced-underground/
References
Al-Ahmad, H., and Gressel, J. (2006). Mitigation using a tandem construct containing a selectively unfit cistron precludes establishment of Brassica napus transgenes in hybrids and backcrosses with weedy Brassica rapa. Plant Biotechnol. J. 4, 23–33. doi: 10.1111/j.1467-7652.2005.00153.ten
PubMed Abstract | CrossRef Full Text | Google Scholar
Bailey, M. R., Woodard, S. Fifty., Callaway, Eastward., Beifuss, Chiliad., Magallanes-Lundback, M., Lane, J. R., et al. (2004). Improved recovery of active recombinant laccase from maize seed. Appl. Microbiol. Biotechnol. 63, 390–397. doi: 10.1007/s00253-003-1362-z
PubMed Abstract | CrossRef Full Text | Google Scholar
Börnke, F., and Broer, I. (2010). Tailoring plant metabolism for the production of novel polymers and platform chemicals. Curr. Opin. Plant Biol. 13, 353–361. doi: ten.1016/j.pbi.2010.01.005
PubMed Abstruse | CrossRef Total Text | Google Scholar
Breyer, D., De Schrijver, A., Goossens, M., Pauwels, K., and Herman, P. (2012). "Biosafety of molecular farming in genetically modified plants," in Molecular Farming in Plants: Contempo Advances and Future Prospects, eds A. Wang and Due south. Ma, (Dordrecht: Springer), 259–274. doi: 10.1007/978-94-007-2217-0_12
CrossRef Full Text | Google Scholar
Burgess, D. G., Ralston, E. J., Hanson, W. G., Heckert, M., Ho, Grand., Jenq, T., et al. (2002). A novel, ii-component system for prison cell lethality and its use in applied science nuclear male-sterility in plants. Plant J. 31, 113–125. doi: x.1046/j.1365-313X.2002.01330.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Celis, C., Scurrah, M., Cowgill, S., Chumbiauca, S., Green, J., and Franco, J. (2004). Environmental biosafety and transgenic spud in a middle of multifariousness for this ingather. Nature 432, 222–225. doi: 10.1038/nature03048
PubMed Abstract | CrossRef Total Text | Google Scholar
Chen, Z., He, Y., Shi, B., and Yang, D. (2013). Human serum albumin from recombinant DNA technology: challenges and strategies. Biochim. Biophys. Acta 2013, 5515–5525. doi: ten.1016/j.bbagen.2013.04.037
PubMed Abstract | CrossRef Full Text | Google Scholar
Clough, R. C., Pappu, 1000., Thompson, K., Beifuss, K., Lane, J., Delaney, D. E., et al. (2006). Manganese peroxidase from the white-rot fungus phanerochaete chrysosporium is enzymatically agile and accumulates to high levels in transgenic maize seed. Institute Biotechnol. J. 4, 53–62. doi: 10.1111/j.1467-7652.2005.00157.x
PubMed Abstruse | CrossRef Full Text | Google Scholar
Commandeur, U., Twyman, R. G., and Fischer, R. (2002). The biosafety of molecular farming in plants. AgBiotechNet 5:ABN10.
Google Scholar
Conner, A. J., Glare, T. R., and Nap, J.-P. (2003). The release of genetically modified crops into the environment, Part Ii. Overview of Ecological Risk Assessment. Found J. 33, 19–46. doi: 10.1046/j.0960-7412.2002.001607.x
PubMed Abstract | CrossRef Total Text | Google Scholar
Fischer, R., Emans, N., Schuster, F., Hellwig, S., and Drossard, J. (1999). Towards molecular farming in the future: using plant-cell-suspension cultures equally bioreactors. Biotechnol. Appl. Biochem. 30, 109–112. doi: ten.1111/j.1470-8744.1999.tb00899.10
PubMed Abstruse | CrossRef Full Text | Google Scholar
Gealy, D. (2005). "Cistron motility betwixt rice (Oryza sativa) and weedy rice (Oryza sativa) — a U.S. Temperate Rice Perspective," in Crop Ferality and Volunteerism, ed. J. Gressel, (Boca Raton, FL: CRC Press), 323–354. doi: 10.1201/9781420037999.ch20
CrossRef Full Text | Google Scholar
Gils, M., Marillonnet, South., Werner, S., Grützner, R., Giritch, A., Engler, C., et al. (2008). A novel hybrid seed system for plants. Institute Biotechnol. J. 6, 226–235. doi: 10.1111/j.1467-7652.2007.00318.x
PubMed Abstract | CrossRef Total Text | Google Scholar
Goldstein, D. A., and Thomas, J. A. (2004). Biopharmaceuticals derived from genetically modified plants. QJM 97, 705–716. doi: 10.1093/qjmed/hch121
CrossRef Full Text | Google Scholar
Gomord, V., Fitchette, A. C., Menu-Bouaouiche, L., Saint-Jore-Dupas, C., Plasson, C., Michaud, D., et al. (2010). Plant-specific glycosylation patterns in the context of therapeutic protein production. Establish Biotechnol. J 8, 564–587. doi: 10.1111/j.1467-7652.2009.00497.x
PubMed Abstract | CrossRef Total Text | Google Scholar
Grabowski, G. A., Golembo, K., and Shaaltiel, Y. (2014). Taliglucerase alfa: an enzyme replacement therapy using plant cell expression technology. Mol. Genet. Metab. 112, 1–8. doi: 10.1016/j.ymgme.2014.02.011
PubMed Abstract | CrossRef Full Text | Google Scholar
Grabsztunowicz, M., Koskela, M. M., and Mulo, P. (2017). Post-translational modifications in regulation of chloroplast function: recent advances. Front. Plant Sci. 8:240. doi: 10.3389/fpls.2017.00240
PubMed Abstract | CrossRef Full Text | Google Scholar
Guillon, S., Trémouillaux-Guiller, J., Pati, P. K., Rideau, Grand., and Gantet, P. (2006). Hairy root research: recent scenario and heady prospects. Curr. Opin. Plant Biol. ix, 341–346. doi: 10.1016/j.pbi.2006.03.008
PubMed Abstruse | CrossRef Full Text | Google Scholar
Häkkinen, South. T., Raven, N., Henquet, 1000., Laukkanen, M.-50., Anderlei, T., Pitkänen, J.-P., et al. (2014). Molecular farming in tobacco hairy roots by triggering the secretion of a pharmaceutical antibody. Biotechnol. Bioeng. 111, 336–346. doi: 10.1002/bit.25113
PubMed Abstract | CrossRef Full Text | Google Scholar
Hanania, U., Ariel, T., Tekoah, Y., Fux, L., Sheva, M., Gubbay, Y., et al. (2017). Establishment of a tobacco BY2 jail cell line devoid of plant-specific xylose and fucose every bit a platform for the production of biotherapeutic proteins. Institute Biotechnol. J. 15, 1120–1129. doi: 10.1111/pbi.12702
PubMed Abstract | CrossRef Full Text | Google Scholar
Hasan, M. M., Kim, H. South., Jeon, J. H., Kim, Southward. H., Moon, B. K., and Song, J. Y. (2014). Metabolic engineering of nicotiana benthamiana for the increased production of taxadiene. Plant Jail cell Rep. 33, 895–904. doi: x.1007/s00299-014-1568-ix
PubMed Abstract | CrossRef Full Text | Google Scholar
He, Y., Ning, T., Xie, T., Qiu, Q., Zhang, L., Dominicus, Y., et al. (2011). Large-scale product of functional human serum albumin from transgenic rice seeds. Proc. Natl. Acad. Sci. U.S.A. 108, 19078–19083. doi: 10.1073/pnas.1109736108
PubMed Abstruse | CrossRef Total Text | Google Scholar
He, Y., Wang, K., and Yan, Due north. (2014). The recombinant expression systems for structure determination of eukaryotic membrane proteins. Poly peptide Cell 5, 658–672. doi: ten.1007/s13238-014-0086-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Hileman, B. (2003). ProdiGene and StarLink incidents provide ammunition to critics. Chem. Eng. News 81, 25–33.
Google Scholar
Hood, E. Due east., and Requesens, D. V. (eds) (2014). "Commercial plant-produced recombinant cellulases for biomass conversion," in Biotechnology in Agriculture and Forestry, Vol. 68(Berlin: Springer), 231–246. doi: ten.1007/978-3-662-43836-7_12
CrossRef Total Text | Google Scholar
Howat, South., Park, B., Oh, I. S., Jin, Y. W., Lee, E. K., and Loake, One thousand. J. (2014). Paclitaxel: biosynthesis, production and future prospects. N. Biotechnol 31, 242–245. doi: 10.1016/j.nbt.2014.02.010
PubMed Abstract | CrossRef Full Text | Google Scholar
Hu, Z., Ding, X., Hu, S., Sun, Y., and Xia, L. (2013). Tissue-specifically regulated site-specific excision of selectable marker genes in bivalent insecticidal, genetically-modified rice. Biotechnol. Lett. 35, 2177–2183. doi: 10.1007/s10529-013-1310-7
PubMed Abstruse | CrossRef Full Text | Google Scholar
Huafang Lai, Q. C., and Jake Stahnke, J. H. (2013). Agroinfiltration as an effective and scalable strategy of gene delivery for production of pharmaceutical proteins. Adv. Tech. Biol. Med. i:103. doi: ten.4172/atbm.1000103
PubMed Abstract | CrossRef Total Text | Google Scholar
Kallolimath, South., Castilho, A., Strasser, R., Grünwald-Gruber, C., Altmann, F., Strubl, South., et al. (2016). Engineering of circuitous protein sialylation in plants. Proc. Natl. Acad. Sci. The statesA. 113, 9498–9503. doi: x.1073/pnas.1604371113
PubMed Abstract | CrossRef Full Text | Google Scholar
Kwit, C., Moon, H. S., Warwick, Due south. I., and Stewart, C. Northward. (2011). Transgene introgression in crop relatives: molecular evidence and mitigation strategies. Trends Biotechnol. 29, 284–293. doi: x.1016/j.tibtech.2011.02.003
PubMed Abstract | CrossRef Full Text | Google Scholar
Final, R. L., Bissinger, P. H., Mahoney, D. J., Radwanski, E. R., and Fink, G. R. (1991). Tryptophan mutants in Arabidopsis: the consequences of duplicated tryptophan synthase B genes. Plant Cell 3, 345. doi: 10.2307/3869210
CrossRef Full Text | Google Scholar
Lu, Y., Rijzaani, H., Karcher, D., Ruf, South., and Bock, R. (2013). Efficient metabolic pathway applied science in transgenic tobacco and tomato plastids with synthetic multigene operons. Proc. Natl. Acad. Sci. U.S.A. 110, E623–E632. doi: 10.1073/pnas.1216898110
PubMed Abstract | CrossRef Total Text | Google Scholar
Malhotra, One thousand., Subramaniyan, G., Rawat, One thousand., Kalamuddin, One thousand., Qureshi, M. I., and Malhotra, P. (2016). Compartmentalized etabolic engineering for Artemisinin biosynthesis and effective malaria treatment by oral delivery of institute cells. Mol. Found 9, 1464–1477. doi: x.1016/j.molp.2016.09.013
PubMed Abstract | CrossRef Full Text | Google Scholar
Mallory-Smith, C. A., and Sanchez Olguin, E. (2011). Gene catamenia from herbicide-resistant crops: it'south not just for transgenes. J. Agric. Food Chem. 59, 5813–5818. doi: 10.1021/jf103389v
PubMed Abstract | CrossRef Full Text | Google Scholar
Mandell, D. J., Lajoie, M. J., Mee, 1000. T., Takeuchi, R., Kuznetsov, G., Norville, J. Due east., et al. (2015). Biocontainment of genetically modified organisms by constructed poly peptide pattern. Nature 518, 55–lx. doi: 10.1038/nature14121
PubMed Abstruse | CrossRef Total Text | Google Scholar
Mariani, C., De Beuckeleer, M., Truettner, J., Leemans, J., and Goldberg, R. B. (1990). Consecration of male sterility in plants by a chimaeric ribonuclease gene. Nature 347, 737–741. doi: x.1038/347737a0
CrossRef Total Text | Google Scholar
Marillonnet, South., Thoeringer, C., Kandzia, R., Klimyuk, Five., and Gleba, Y. (2005). Systemic Agrobacterium Tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat. Biotechnol. 23, 718–723. doi: 10.1038/nbt1094
PubMed Abstract | CrossRef Full Text | Google Scholar
Maselko, Chiliad., Heinsch, S. C., Chacón, J. M., Harcombe, Westward. R., and Smanski, Thousand. J. (2017). Engineering science species-like barriers to sexual reproduction. Nat. Commun. 8:883. doi: ten.1038/s41467-017-01007-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Mercx, Southward., Smargiasso, Northward., Chaumont, F., De Pauw, East., Boutry, Thousand., and Navarre, C. (2017). Inactivation of the β(one,two)-Xylosyltransferase and the α(1,3)-Fucosyltransferase Genes in Nicotiana Tabacum BY-2 Cells by a Multiplex CRISPR/Cas9 Strategy Results in Glycoproteins without Found-Specific Glycans. Front. Institute Sci. 8:403. doi: ten.3389/fpls.2017.00403
PubMed Abstract | CrossRef Total Text | Google Scholar
Michael, P. J., Owen, M. J., and Powles, South. B. (2010). Herbicide-resistant weed seeds contaminate grain sown in the western Australian Grainbelt. Weed Sci. 58, 466–472. doi: 10.1614/ws-d-09-00082.ane
CrossRef Full Text | Google Scholar
Moon, H. S., Abercrombie, Fifty. L., Eda, South., Blanvillain, R., Thomson, J. G., Ow, D. Westward., et al. (2011). Transgene excision in pollen using a Codon optimized serine resolvase CinH-RS2 site-specific recombination system. Plant Mol. Biol. 75, 621–631. doi: 10.1007/s11103-011-9756-ii
PubMed Abstract | CrossRef Full Text | Google Scholar
Nguyen, L. V., Cox, G. Yard., Ke, J. Due south., Peele, C. G., and Dickey, L. F. (2012). Genetic engineering of a lemna isoleucine auxotroph. Transgenic Res. 21, 1071–1083. doi: 10.1007/s11248-012-9594-ii
PubMed Abstract | CrossRef Full Text | Google Scholar
Ohmori, South., Tabuchi, H., Yatou, O., and Yoshida, H. (2012). Agronomic traits and gene containment capability of cleistogamous rice lines with the superwoman1-cleistogamy mutation. Brood. Sci. 62, 124–132. doi: 10.1270/jsbbs.62.124
PubMed Abstruse | CrossRef Full Text | Google Scholar
Pammer, J., Weninger, W., and Tschachler, Due east. (1998). Human keratinocytes express cellular Prion-related protein in vitro and during inflammatory skin diseases. Am. J. Pathol. 153, 1353–1358. doi: ten.1016/S0002-9440(10)65720-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Que, Q., Elumalai, S., Li, X., Zhong, H., Nalapalli, S., Schweiner, M., et al. (2014). Maize transformation applied science development for commercial issue generation. Front end. Found Sci. five:379. doi: 10.3389/fpls.2014.00379
PubMed Abstruse | CrossRef Total Text | Google Scholar
Reeves, R. G., Bryk, J., Altrock, P. M., Denton, J. A., and Reed, F. A. (2014). First steps towards underdominant genetic transformation of insect populations. PLoS I 9:e97557. doi: 10.1371/journal.pone.0097557
PubMed Abstruse | CrossRef Full Text | Google Scholar
Reichman, J. R., Watrud, 50. S., Lee, E. H., Burdick, C. A., Bollman, M. A., and Storm, K. (2006). Establishment of transgenic herbicide-resistant creeping bentgrass (Agrostis stolonifera Fifty.) in nonagronomic habitats. Mol. Ecol. fifteen, 4243–4255. doi: ten.1111/j.1365-294X.2006.03072.ten
PubMed Abstract | CrossRef Total Text | Google Scholar
Ruf, S., Hermann, M., Berger, I. J., Carrer, H., and Bock, R. (2001). Stable genetic transformation of tomato plastids and expression of a foreign poly peptide in fruit. Nat. Biotechnol. 19, 870–875. doi: x.1038/nbt0901-870
PubMed Abstract | CrossRef Full Text | Google Scholar
Santos, R. B., Abranches, R., Fischer, R., Sack, M., and The netherlands, T. (2016). Putting the spotlight back on plant break cultures. Front. Plant Sci. 7:297. doi: 10.3389/fpls.2016.00297
PubMed Abstract | CrossRef Full Text | Google Scholar
Sayre, R., Beeching, J. R., Cahoon, E. B., Egesi, C., Fauquet, C., Fellman, J., et al. (2011). The BioCassava plus program: biofortification of cassava for sub-saharan Africa. Annu. Rev. Plant Biol. 62, 251–272. doi: 10.1146/annurev-arplant-042110-103751
PubMed Abstruse | CrossRef Full Text | Google Scholar
Shen, B., Sun, X., Zuo, X., Shilling, T., Apgar, J., Ross, M., et al. (2012). Applied science a thermoregulated intein-modified xylanase into maize for consolidated lignocellulosic biomass processing. Nat. Biotechnol. 30, 1131–1136. doi: 10.1038/nbt.2402
PubMed Abstract | CrossRef Total Text | Google Scholar
Stein, H., Wilensky, M., Tsafrir, Y., Rosenthal, M., Amir, R., and Avraham, T. (2009). Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules 10, 2640–2645. doi: 10.1021/bm900571b
PubMed Abstract | CrossRef Total Text | Google Scholar
Stoger, E., Fischer, R., Moloney, M., and Ma, J. Grand.-C. (2014). Plant molecular pharming for the treatment of chronic and infectious diseases. Annu. Rev. Plant Biol. 65, 743–768. doi: 10.1146/annurev-arplant-050213-035850
PubMed Abstruse | CrossRef Full Text | Google Scholar
Svab, Z., and Maliga, P. (2007). Exceptional transmission of plastids and mitochondria from the transplastomic pollen parent and its affect on transgene containment. Proc. Natl. Acad. Sci. United states of americaA. 104, 7003–7008. doi: 10.1073/pnas.0700063104
PubMed Abstruse | CrossRef Full Text | Google Scholar
Thayer, A. (2002). USDA fines prodigene; manufacture reacts to subcontract interests. Chem. Eng. News 80:12. doi: 10.1021/cen-v080n050.p012a
CrossRef Total Text | Google Scholar
Van Der Maarel, Thou. J., Van Der Veen, B., Uitdehaag, J. C. One thousand., Leemhuis, H., and Dijkhuizen, 50. (2002). Properties and applications of starch-converting enzymes of the α-amylase family unit. J. Biotechnol. 94, 137–155. doi: ten.1016/S0168-1656(01)00407-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, A., and Ma, S. (2012). Molecular Farming in Plants: Recent Advances and Future Prospects. Dordrecht: Springer, doi: 10.1007/978-94-007-2217-0
CrossRef Full Text | Google Scholar
Wani, S. H., Sah, S. K., Sági, L., and Solymosi, G. (2015). Transplastomic plants for innovations in agronomics. A review. Agron. Sustain. Dev. 35, 1391–1430. doi: 10.1007/s13593-015-0310-five
CrossRef Full Text | Google Scholar
Waters, A. J., Capriotti, P., Gaboriau, D. C. A., Papathanos, P. A., and Windbichler, N. (2018). Rationally-engineered reproductive barriers using CRISPR CRISPRa: an evaluation of the constructed species concept in Drosophila melanogaster. Sci. Rep. 8:13125. doi: 10.1038/s41598-018-31433-2
PubMed Abstract | CrossRef Total Text | Google Scholar
Wurtzel, E. T., Vickers, C. E., Hanson, A. D., Millar, A. H., Cooper, M., and Voss-Fels, K. (2019). Revolutionizing agriculture with constructed biological science. Nat. Plants 5, 1207–1210. doi: 10.1038/s41477-019-0539-0
PubMed Abstract | CrossRef Total Text | Google Scholar
Yao, J., Weng, Y., Dickey, A., and Wang, K. Y. (2015). Plants as factories for human pharmaceuticals: applications and challenges. Int. J. Mol. Sci 16, 28549–28565. doi: x.3390/ijms161226122
PubMed Abstract | CrossRef Total Text | Google Scholar
Yusibov, V., Rabindran, S., Commandeur, U., Twyman, R. Thousand., and Fischer, R. (2006). The potential of plant virus vectors for vaccine production. Drugs R D seven, 203–217. doi: x.2165/00126839-200607040-00001
PubMed Abstract | CrossRef Full Text | Google Scholar
Zeugolis, D. I., Li, B., Lareu, R. R., Chan, C. K., and Raghunath, Chiliad. (2008). Collagen solubility testing, a quality assurance footstep for reproducible electro-spun nano-fibre fabrication. A technical notation. J. Biomater. Sci. Polym. Ed. xix, 1307–1317. doi: 10.1163/156856208786052344
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, C., Norris-Caneda, M. H., Rottmann, W. H., Gulledge, J. E., Chang, S., Kwan, B. Y. H., et al. (2012). Control of pollen-mediated cistron flow in transgenic copse. Institute Physiol. 159, 1319–1334. doi: 10.1104/pp.112.197228
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, D., VanFossen, A. L., Pagano, R. M., Johnson, J. Due south., Parker, 1000. H., Pan, Due south., et al. (2011). Consolidated pretreatment and hydrolysis of establish biomass expressing cell wall degrading enzymes. Bioenergy Res. 4, 276–286. doi: x.1007/s12155-011-9138-2
PubMed Abstruse | CrossRef Full Text | Google Scholar
Zhang, 50., Jing, F., Li, F., Li, M., Wang, Y., and Wang, G. (2009). Development of transgenic Artemisia annua (Chinese Wormwood) plants with an enhanced content of artemisinin, an effective anti-malarial drug, past hairpin-RNA-mediated gene silencing. Biotechnol. Appl. Biochem. 52:199. doi: 10.1042/ba20080068
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, X., Buehner, Due north. A., Hutson, A. M., Estes, M. K., and Mason, H. Southward. (2006). Tomato is a highly constructive vehicle for expression and oral immunization with Norwalk virus capsid protein. Plant Biotechnol. J. four, 419–432. doi: 10.1111/j.1467-7652.2006.00191.x
PubMed Abstruse | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fpls.2020.00210/full
Posted by: kiddmembech.blogspot.com

0 Response to "What Is Fundamentally Different Between Generating Transgenic Animals Versus Transgenic Plants?"
Post a Comment